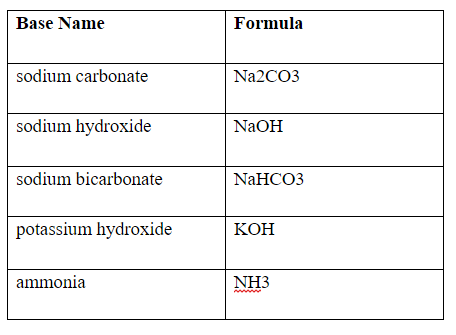
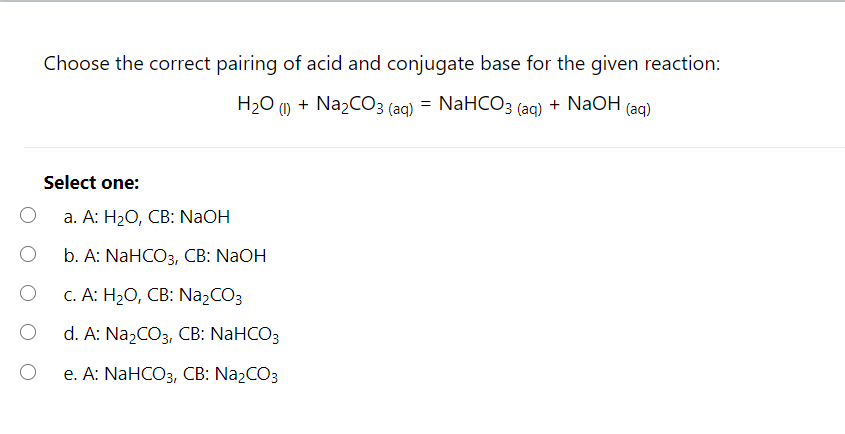Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram

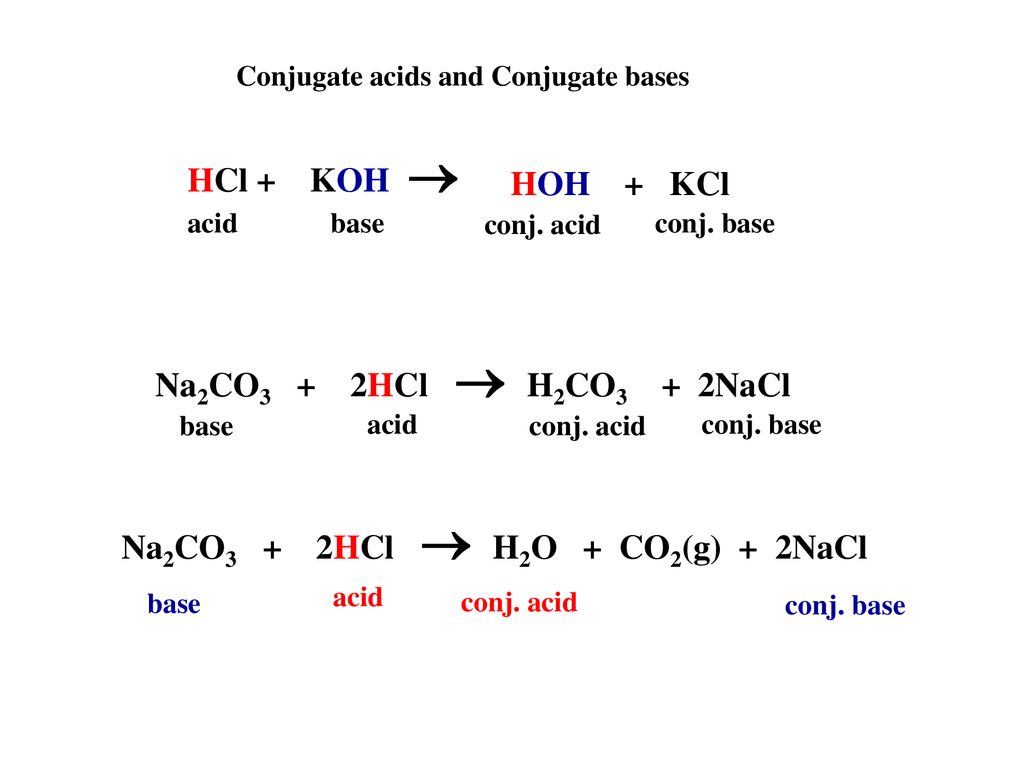
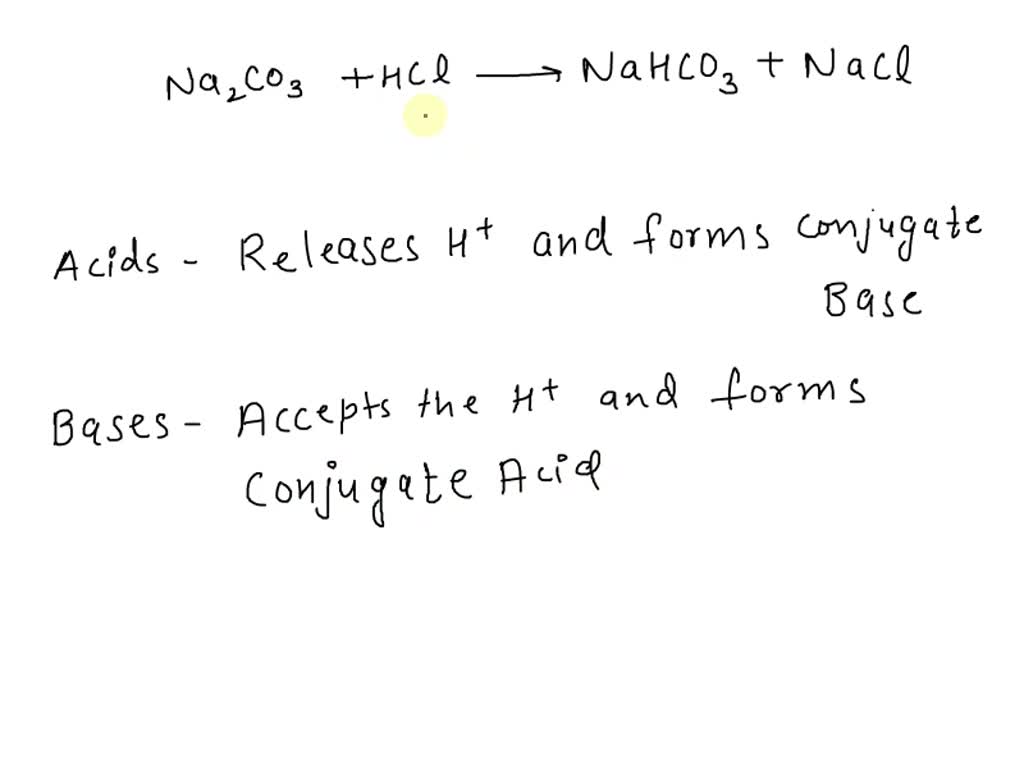
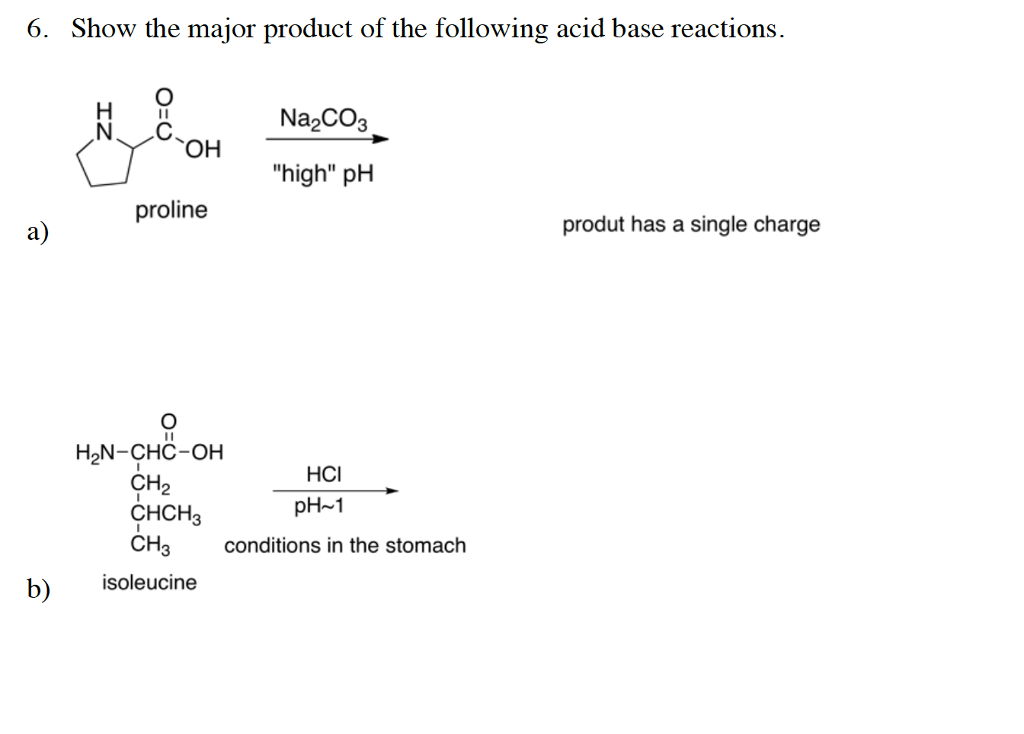
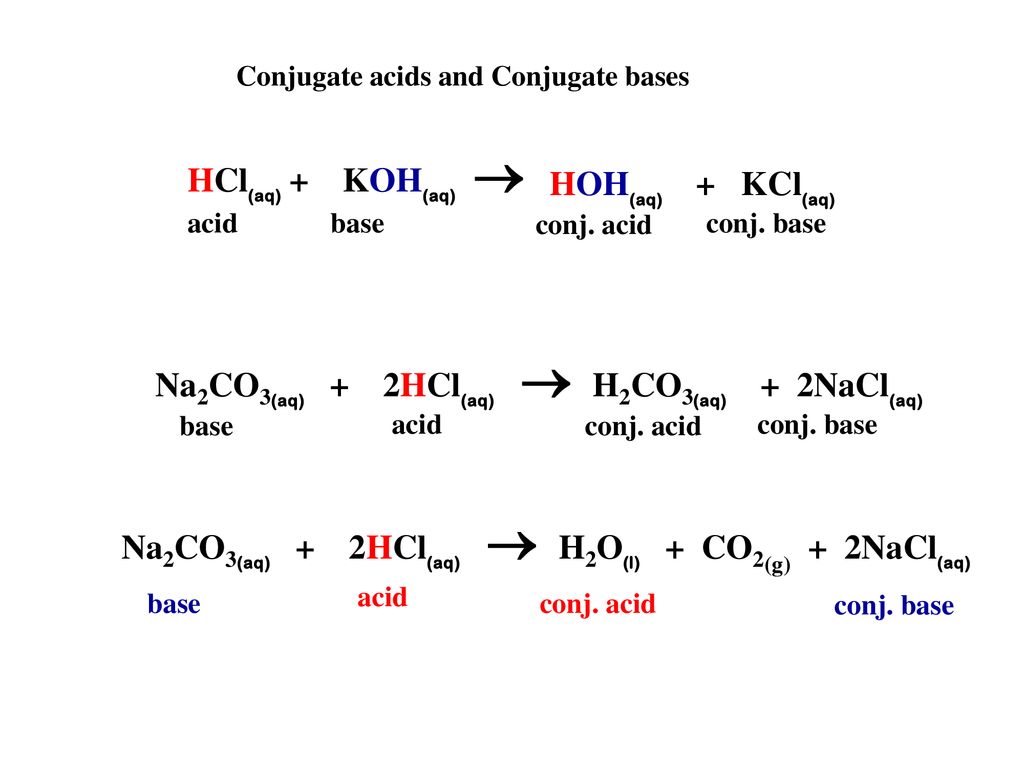
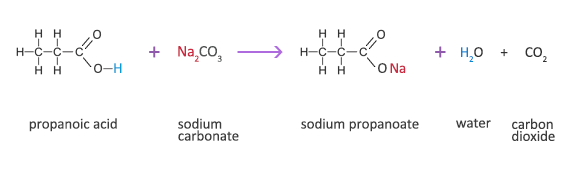
Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

The titration of Na2CO3 with HCl has the following qualitative profile: a. Identify the major species in solution as points A-F. b. For the titration of 25.00 mL of 0.100 M Na2CO3