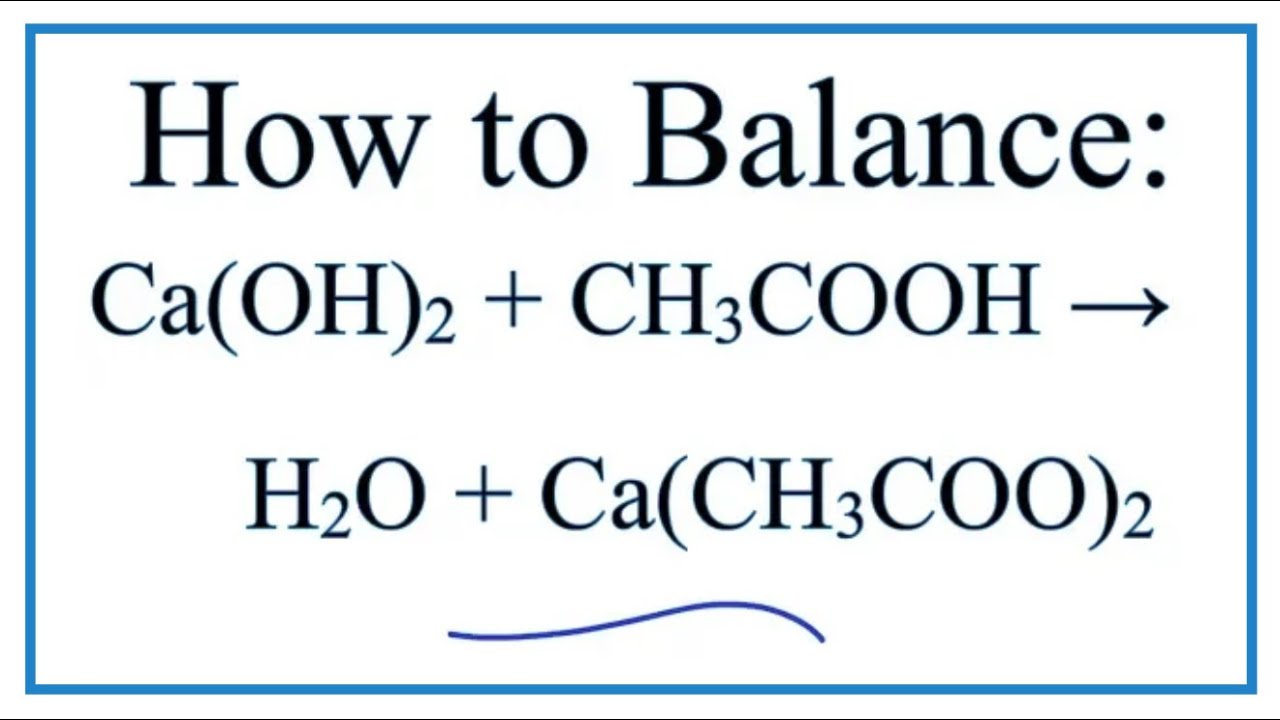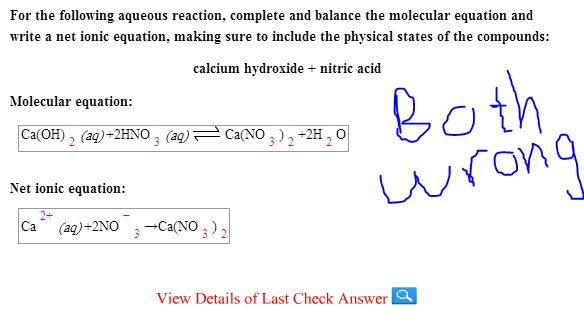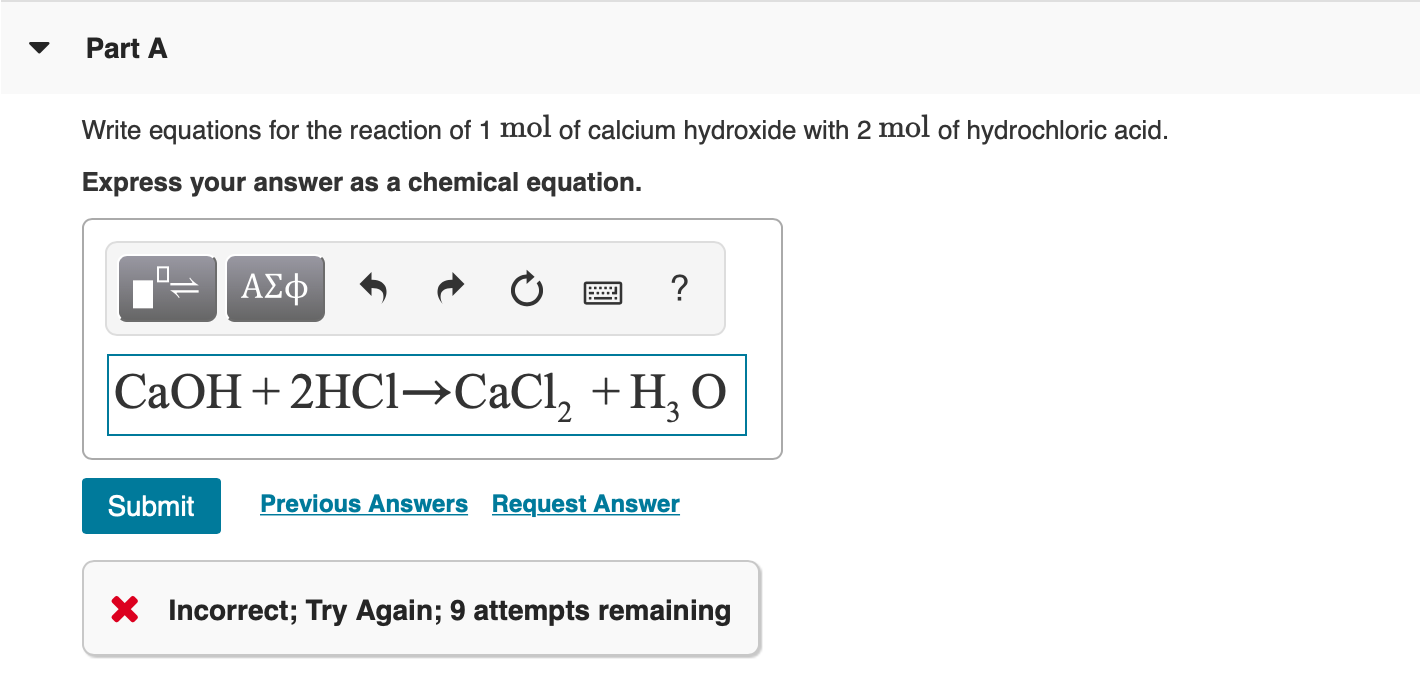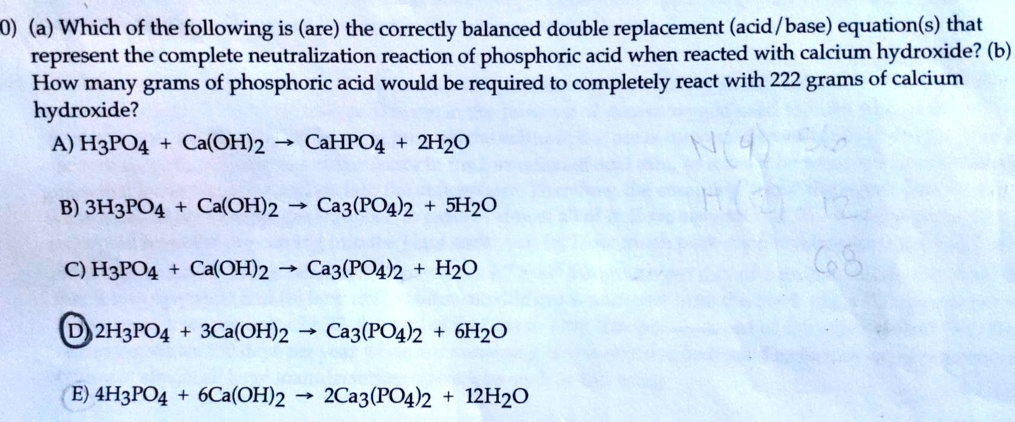
SOLVED: 0) (a) Which of the following is (are) the correctly balanced double replacement (acid /base) equation(s) that represent the complete neutralization reaction of phosphoric acid when reacted with calcium hydroxide? (b)

SOLVED: 9 . Write a balanced neutralization reaction between the following acids and bases a. Carbonic acid and Potassium hydroxide Phosphoric acid and Calcium hydroxide c. Nitric acid and Aluminum hydroxide d.

ACIDS & BASES module i.An acid is a chemical substance that …………………in water to produce ………………. ions. ii.A base is a chemical substance that ………………in. - ppt download

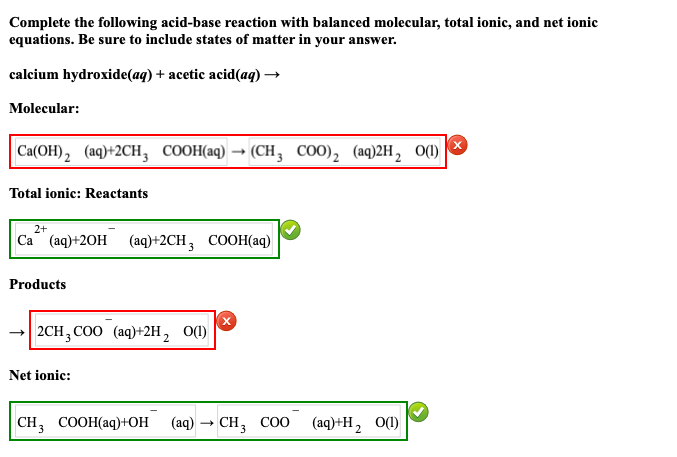
SOLVED: Be sure to answer all parts: Complete the following acid-base reaction with balanced molecular; total ionic, and net ionic equations. Be sure to include states of matter in your answer: calcium
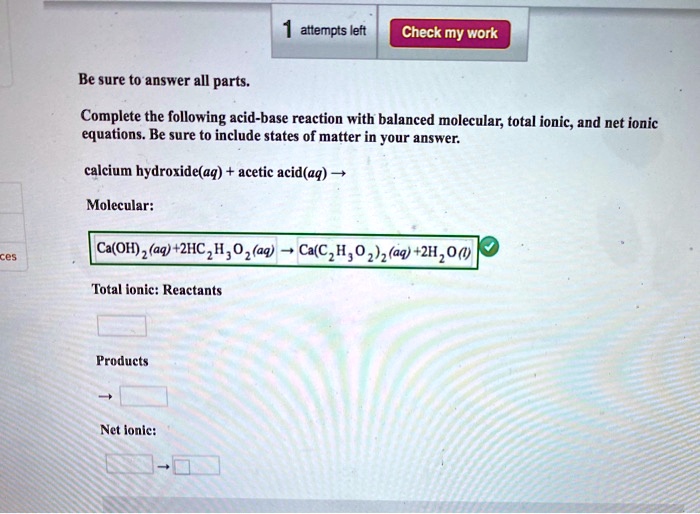
SOLVED: attempts left Check my work Be sure to answer all parts. Complete the following acid-base reaction with balanced molecular; total ionic, and net ionic equations. Be sure to include states of

Introduction to Acids and Bases. Acid A substance that produces hydrogen ions, H + (aq), when it dissolves in water. Sour-tasting and good conductors. - ppt download